It’s late at night in Munich, Germany sometime in the late 2000’s. A man huddles in a dimly lit basement, painting. He paints at night after a complaint from the professors upstairs about the fumes during their lectures. The ultimate result will be a heavy, rectangular slab with a shiny black finish. This black hunk of metal represents several years of research done by two Ph.D. students. Their project, a union of biology and physics, isn’t just about to help them graduate, but is about to launch a revolution in the field of protein biophysics.
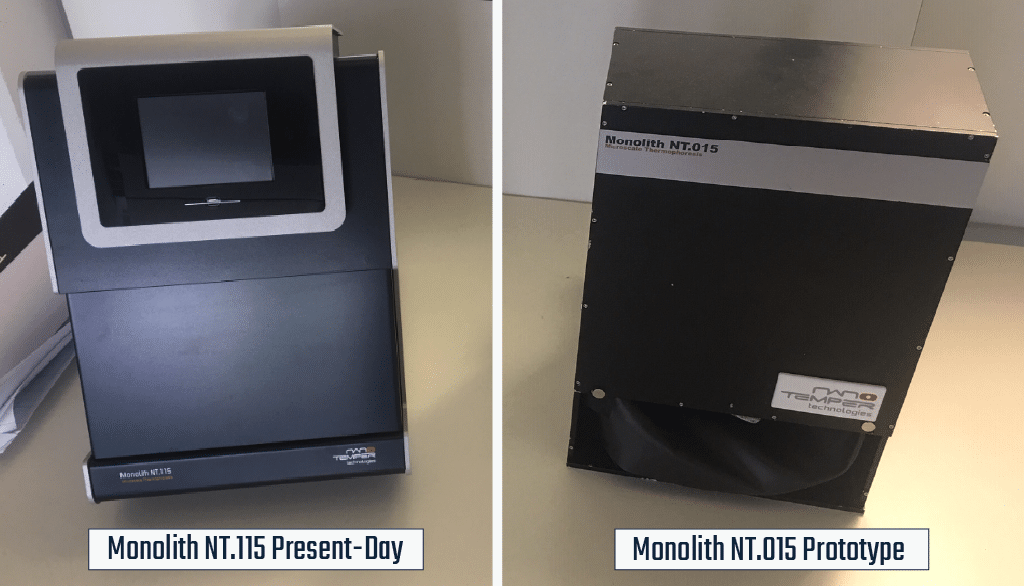
The first NanoTemper instrument
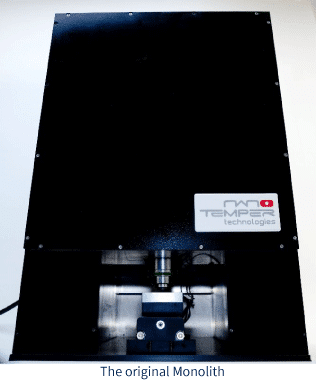
This black box contained the technology that led to NanoTemper: MicroScale Thermophoresis (MST for short). MST measures the interaction between molecules using principles defined by Carl Ludwig and Charles Soret over a century and a half ago. While the principles were explained in the mid-1800s, it wasn’t until our CEOs, Philipp Baaske and Stefan Duhr, were working as Ph.D. students at Ludwig Maximilian University of Munich and found a way to apply the theory of thermophoresis to practical applications measuring biological interactions. The rest is history.
The original MST measurements weren’t done in a dedicated instrument, but rather on a confocal microscope. However, when the principle proved sound, the idea of constructing a dedicated instrument began and a prototype (shown above) was made. They spent the next several years refining the prototype and applying for business grants to get started producing the instruments.
But all that hard work would go for naught if they couldn’t get the instruments to make a splash among scientists. What better way to make a big impact than having a good name? A good name can have profound staying power in the minds of consumers— how often do you say you’re going to Google something, blow your nose in a Kleenex, or drive your Zamboni? (Okay, maybe the last one is a little niche.) So as NanoTemper has assembled a line of instruments dedicated to improving the lives of protein researchers, each one of our instruments has been named thoughtfully.
What’s in a name?
Creating a brand-new instrument is a labor of love, and all that time and energy can make it feel as auspicious as naming a child. Giving it an identity by giving it a name is a proud and profound moment of discovery, not to mention a fun way to show off your personality.
Fans of Sci-fi and/or Kubrick will notice something familiar about the prototype pictured above:

The original MST device looked an awful lot like the monoliths that appear in 2001: Space Odyssey. Not only was this physical similarity obvious to our Sci-fi fan CEO, Philipp, but there was another element to the Monolith in the novel: it was a seed that sparked the development of technology and advancement of the human race. Lofty goals perhaps for such a small benchtop device, but its creators, our CEOs, truly believed the Monolith would go on to change how scientists measured biophysical interactions. Their dedication to the technology helped recruit others to their cause, including Gernot Theissig, who also played an instrumental role in developing the prototype.
But where does the NT.115 come from? NT stands for NanoTemper (as you might imagine) and the 115 has to do with the evolution of the final product. Originally, the prototype Monolith had slots for 15 capillaries. As the instrument was refined, it made sense to add an extra slot., Furthermore, instead of calling it the NT.16, it became the NT.1+15, or NT.115. Why?
According to Phillip, “Higher numbers just sound better, plus it kind of reminded us of the Terminator T-101.”
Once the Monolith launched, the rest is history. Today we have hundreds of instruments across the globe, and our team of dedicated scientists has expanded well past three guys in a basement. However, if you ever find yourself in that basement room at LMU, look around at the walls—you might just find some familiar-looking black paint.





















