Your guide to Quality by Design – part 2: efficient and compliant biologics development with PrometheusTM Panta C
Introduction: the role of data precision in later-stage QbD
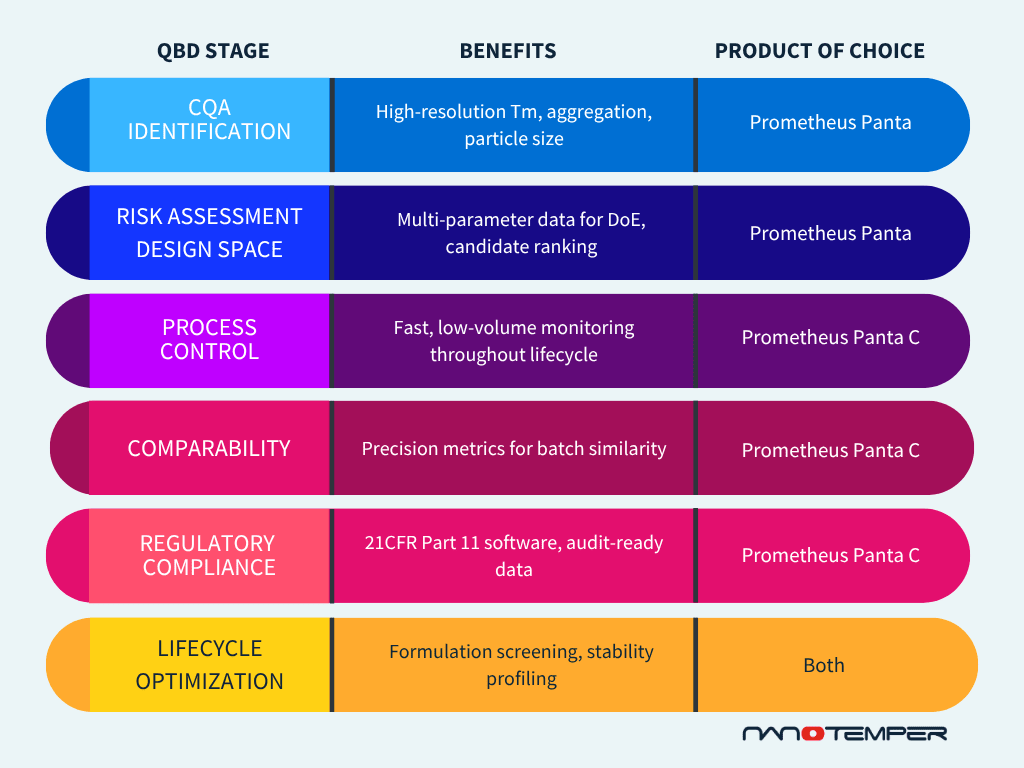
Building on part 1’s focus on early-stage CQA definition and design space exploration, this second blog dives deeper into how Quality by Design (QbD) principles guide later-stage activities in biologics development. These include process control, comparability assessment, and lifecycle management – all areas where precision and data integrity become critical.
Here, the Prometheus Panta C, the newest addition to the Panta product line, powered by Multiplexing Optical Methods (MOM™), plays a central role in supporting compliant and high-performing biopharmaceutical pipelines.
3. Process development and control strategy: staying within the design space
As biologics move from R&D to pilot and commercial scale, maintaining control over CQAs becomes essential.
Prometheus Panta C offers a practical solution for monitoring molecular behavior at various development stages:
- Low sample volumes and turnaround times (<2 hours) enable frequent in-process checks
- Real-time feedback supports tight control strategies
- High-resolution aggregation and stability data help proactively detect deviations
These capabilities are aligned with the QbD mandate to control quality through process understanding – not just end-point testing.
4. Demonstrating comparability and meeting regulatory expectations
Regulatory agencies require strong evidence that process changes or scale-up do not affect product quality. Demonstrating comparability is a regulatory necessity, and a scientific challenge.
Prometheus Panta C supports this with:
- High precision (±0.1 °C Tm) for comparability studies
- 21 CFR part 11-compliant software, including audit trails, electronic signatures, and user access control
- Reproducible multi-parameter data sets to demonstrate batch-to-batch and site-to-site consistency
This data integrity and reproducibility build trust, both internally and with regulators.
5. Enabling lifecycle management with high-resolution biophysical data
QbD doesn’t end at approval. It evolves over a product’s lifecycle, requiring tools that adapt and support post-approval changes, formulation optimization, and continuous improvement.
Prometheus Panta and Panta C contribute by:
- Supporting buffer and excipient screening to enhance stability
- Enabling process comparability assessments during technology transfer or site changes
- Providing forced degradation profiles to support shelf-life assignment and regulatory submissions
This long-term analytical support ensures biologics remain robust, safe, and effective, even as manufacturing processes evolve.
Conclusion: a perfect fit for QbD-driven biologics
Quality by Design has transformed biologics development by embedding quality deep into process and product understanding. With its unparalleled multi-parameter characterization, high precision, and regulatory-ready data handling, the Prometheus Panta product line becomes more than an suite of instruments: it’s a strategic enabler of QbD.
By leveraging Multiplexing Optical Methods in both Prometheus Panta and Prometheus Panta C, throughout the entire development cycle, scientists can systematically minimize risk, optimize processes, ensure product consistency, and meet regulatory expectations with confidence, paving the way for safer, more effective biologics delivered to patients faster.